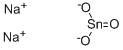Sodium Stannate
- CAS No. :12058-66-1
- Molecular Formula:Na2O3Sn
- Purity:Reagent Grade
- Molecular Weight:212.69
Product Details
pd_meltingpoint:140 °C
Packing:100g/Bag
Appearance:White Powder
Product Description
Sodium stannate, formally sodium hexahydroxostannate(IV), is the inorganic compound with the formula Na2[Sn(OH)6]. This colourless salt forms upon dissolving metallic tin or tin(IV) oxide in sodium hydroxide, and is used as a stabiliser for hydrogen peroxide.In older literature, stannates are sometimes represented as having the simple oxyanion SnO32−, in which case this compound is sometimes named as sodium stannate–3–water and represented as Na2SnO3·3H2O, a hydrate with three waters of crystallisation.The anhydrous form of sodium stannate, Na2SnO3, is recognised as a distinct compound with its own CAS Registry Number, 12058-66-1 , and a distinct material safety data sheet.
Sodium Stannate (synonyms: Sodium tin(IV) oxide, sodium hexahydroxostannate) is an inorganic metal oxide containing alkali metal sodium from group IA and post transition metal tin from group IVA in +4 oxidation state. Sodium Stannate is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. Additional technical, research and safety information is available.
Alkali metal stannate compounds are prepared by dissolving elemental tin in a suitable metal hydroxide, in the case of sodium stannate by the reaction:
Sn + 2 NaOH + 4 H2O → Na2[Sn(OH)6] + 2 H2
A similar reaction occurs when tin dioxide is dissolved in base:
SnO2 + 2 NaOH + 2 H2O → Na2[Sn(OH)6]
The anhydrous form can also be prepared from tin dioxide by roasting with sodium carbonate in a mixed carbon monoxide / carbon dioxide environment:
SnO2 + Na2CO3 → Na2SnO3 + CO2
The anion is a coordination complex that is octahedral in shape, similar to most stannates, such as the hexachlorostannate anion [SnCl6]2−. The Sn—O bond distances average 2.071 Å.
Application
Sodium stannate is used in beam medium, fabric fire retardant, tin plating. Mainly used in alkaline tin plating and copper tin alloy plating industry. Used in textile industry as fire retardant and weight increasing agent. The dye industry uses mordants. Also used for dyeing, ceramics, glass, stabilizer for hydrogen peroxide, blueprint paper, laboratory reagent and other industries.
Notes: Sodium stannate just for research use only. Not for diagnostic or therapeutic use. Not for human use.
Specifications
| Chemical Name or Material | Sodium Stannate |
| Synonyms | DI-SODIUM TIN TRIOXIDE;SODIUM TIN(IV) OXIDE;SODIUM M-STANNATE;SODIUM STANNATE;disodiumstannate;Stannic acid sodium salt;Sodium stannate 42-45% SnO2 basis;Stannate (SnO32-),sodium |
| CAS # | 12058-66-1 |
| IUPAC Name | disodium; dioxido(oxo)tin |
| Molecular Formula/ Molecular Weight |
Na2O3Sn=212.69 |
| Melting Point | 140 °C |
| Density | 4.68 g/cm3(Temp: 25 °C) |
| Solubility Information | Slightly soluble in water |
| Storage Temp. | 2-8 °C |
| Colour & Form | Colourless Crystalline Or White Powder |
| EC No. | 235-030-5 |
| Bulk Density | 7.3 g/cm3 |
| Exact Mass | 213.866477 g/mol |
| SMILES | [O-][Sn](=O)[O-].[Na+].[Na+] |
| InchI Identifier | InChI=1S/2Na.3O.Sn/q2*+1;;2*-1; |
| InChI key | TVQLLNFANZSCGY-UHFFFAOYSA-N |
References
"Material Safety Data Sheet – sodium stannate trihydrate MSDS". Science Lab. 21 May 2013. Retrieved 1 June 2017.
Clark, John D. (1972). Ignition! An Informal History of Liquid Rocket Propellants. Rutgers University Press. ISBN 0813507251.
Similarly, stannites are sometimes represented with the anion SnO22−
National Center for Biotechnology Information (2017). "Sodium Stannate". PubChem. Retrieved 1 June 2017. "Sodium Stannate MSDS" (PDF). Santa Cruz Biotechnology. 14 June 2011. Retrieved 1 June 2017.
Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0750633654.
Zhang, Yuanbo; Su, Zijian; Liu, Bingbing; You, Zhixiong; Yang, Guang; Li, Guanghui; Jiang, Tao (2014). "Sodium stannate preparation from stannic oxide by a novel soda roasting–leaching process". Hydrometallurgy. 146: 82–88. doi:10.1016/j.hydromet.2014.03.008.
Jacobs, Herbert; Stahl, Rainer (2000). "Neubestimmung der Kristallstrukturen der Hexahydroxometallate Na2Sn(OH)6, K2Sn(OH)6 und K2Pb(OH)6". Z. Anorg. Allg. Chem. (in German). 626 (9): 1863–1866. doi:10.1002/1521-3749(200009)626:9<1863::AID-ZAAC1863>3.0.CO;2-M
Superiority
1.Powerful R & D team
2.The ability of quantized production,from grams to tons;
3.Various kinds of advanced equipment in the laboratory and plant;
4.Strict QC standard to meet the demand of customers,advanced equipment for analysis and a ssay;
5.Efficient and safe logistic plans for chemical products transportation;
6.Excellent after-sale service.
7. Higest quality and good package
If you have any demands,you are welcome to contact us at any time,we are always ready to give you the fastest reply and the best service.