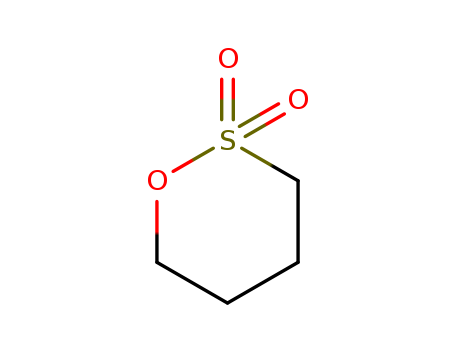
1,4-Butane sultone
- CAS No. :1633-83-6
- Molecular Formula:C<sub>4</sub>H<sub>8</sub>O<sub>3</sub>S
- Purity:
- Molecular Weight:136.172
Product Details
pd_meltingpoint:12-15 °C(lit.)
Appearance:clear colorless to yellowish liquid
Top Quality 1,4-Butane sultone 1633-83-6 Hot Sell In Stock
- Molecular Formula:C4H8O3S
- Molecular Weight:136.172
- Appearance/Colour:Colorless to pale-yellow liquid
- Vapor Pressure:0.00206mmHg at 25°C
- Melting Point:12-15 °C(lit.)
- Refractive Index:n20/D 1.464(lit.)
- Boiling Point:299.9 °C at 760 mmHg
- Flash Point:135.2 °C
- PSA:51.75000
- Density:1.308 g/cm3
- Stability:Stable at room temperature
1,4-Butane sultone(Cas 1633-83-6) Usage
| Sulfo-Alkylating Agent |
1,4-Butane sultone is used to introduce sulfobutyl groups into hydrophobic compounds with nucleophilic functional groups (e.g., hydroxyl or amino groups). |
| Chemical Intermediate | Employed in organic synthesis for the preparation of various chemical products and intermediates. |
| API Production | Used as an intermediate in the production of Active Pharmaceutical Ingredients (APIs). |
InChI:InChI=1/C4H8O3S/c5-8(6)4-2-1-3-7-8/h1-4H2
1633-83-6 Relevant articles
Butane sultone integrated superhydrophilic polyamide membranes for efficient ionic separation
Shabab Hussain a , Zhizhen Ye a b , Xinsheng Peng a b
, Desalination Volume 558, 15 July 2023, 116611
In this work, we first report the chemical integration of 1,4-butane sultone into the PA selective layer on a highly porous hydrophilic PES membrane by one step facile interfacial polymerization process. The BS makes the selective layer of the ion-separation membrane superhydrophilic with recorded negative surface zeta potential and wettability, inheriting the membrane high water transport properties.
Determination of Low-ppm Levels of 1,4-Butane Sultone in Sulfobutyl Ether β-Cyclodextrin Using Liquid–Liquid Extraction and GC–MS
Meng Wang, Hongmei Wen, Sheng Yu, Zhengyu Yan
, Chromatographia 75(15-16), 2012
1,4-Butane sultone is an impurity in sulfobutyl ether β-cyclodextrin (SBE-β-CD), and has been reported to be a weak carcinogen. There is, however, no report of any method of determining 1,4-butane sultone in SBE-β-CD. In this paper, a gas chromatography–mass spectrometry (GC–MS) method was developed for the determination of 1,4-butane sultone. The samples were dissolved in water and extracted with chloroform.
4-Imidazol-1-yl-butane-1-sulfonic acid or a novel liquid salt? The NMR analysis and dual solvent-catalytic efficiency for one-pot synthesis of xanthenes
Nader Ghaffari Khaligh , Lim Shi Teng , Ong Chiu Ling , Mohd Rafie Johan , Juan Joon Ching
, Journal of Molecular Liquids Volume 278, 15 March 2019, Pages 19-32
In summary, the reaction of the equimolar mixture of imidazole and 1,4-butane sultone was performed at 90 °C in ethylbenzene for 12 h and the structure of the product was elucidated by FTIR, 1H and 13C NMR, 1H,1H-COSY, 1H,13C- and 1H,15N-HMBC analysis. Then, the physical properties and pH of the aqueous solution of the new liquid salt were determined and measured. Based on the NMR analysis, the presence of zwitterionic and ionic structures viz.
1633-83-6 Upstream products
-
61161-42-0
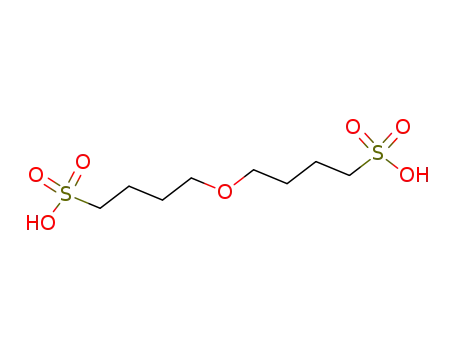
4,4'-oxy-bis-butane-1-sulfonic acid
-
109-69-3
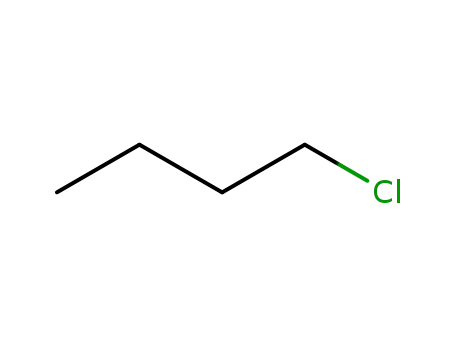
n-Butyl chloride
-
26978-64-3
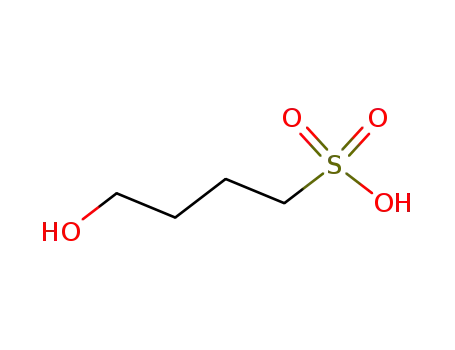
4-hydroxy-butane-1-sulfonic acid
-
109-99-9
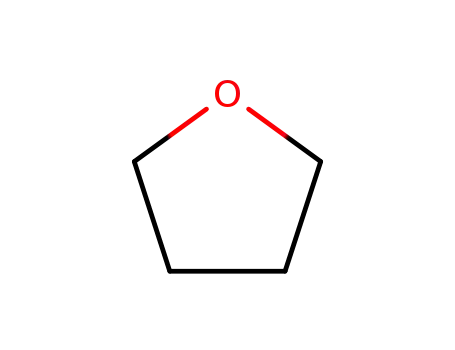
tetrahydrofuran
1633-83-6 Downstream products
-
21876-43-7
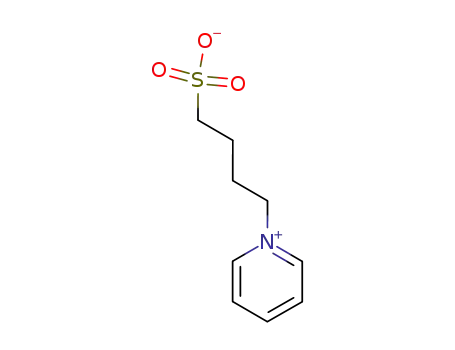
pyridinium propyl sulfobetaine
-
71205-44-2
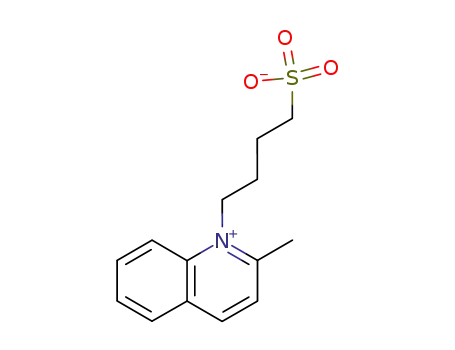
2-methyl-1-(4-sulfonatobutyl)-quinolinium
-
55526-95-9
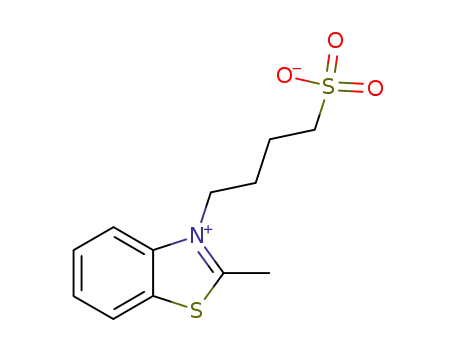
N-(δ-sulfonatobutyl)-2-methyl-benzothiazole
-
3944-72-7
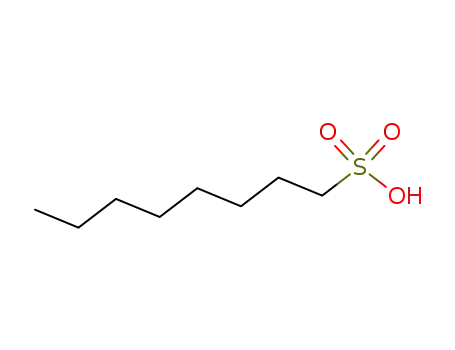
1-octanesulfonic acid