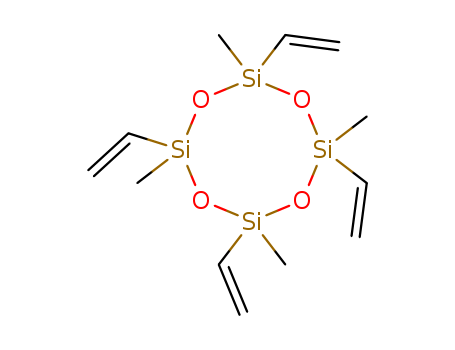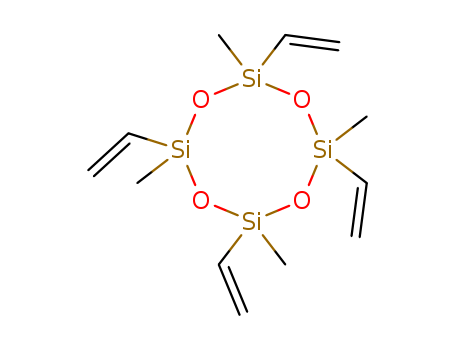
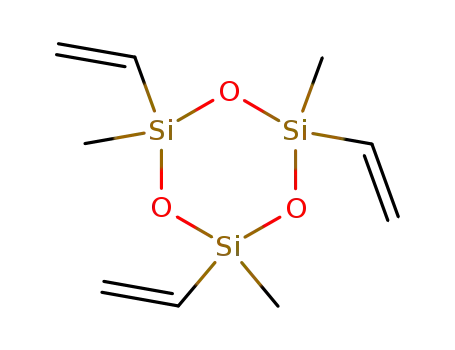
2,4,6,8-Tetravinyl-2,4,6,8-tetramethylcyclotetrasiloxane
- CAS No. :2554-06-5
- Molecular Formula:C<sub>12</sub>H<sub>24</sub>O<sub>4</sub>Si<sub>4</sub>
- Purity:
- Molecular Weight:344.662
Product Details
pd_meltingpoint:-44 °C
Appearance:Colorless transparent liquid
China cas 2554-06-5 factory wholesale 1,3,5,7-Tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane(VMC) at affordable price
- Molecular Formula:C12H24O4Si4
- Molecular Weight:344.662
- Appearance/Colour:Colorless transparent liquid
- Vapor Pressure:0.04mmHg at 25°C
- Melting Point:-44 °C
- Refractive Index:n20/D 1.435
- Boiling Point:247.805 °C at 760 mmHg
- Flash Point:94.338 °C
- PSA:36.92000
- Density:0.967 g/cm3
- LogP:3.25520
2,4,6,8-Tetravinyl-2,4,6,8-tetramethylcyclotetrasiloxane(Cas 2554-06-5) Usage
|
Hazard |
Low toxicity by ingestion and inhalation. |
|
Flammability and Explosibility |
Nonflammable |
|
Purification Methods |
A 7mL sample can be distilled in a small Vigreux column (p 11) at atmospheric pressure without polymerisation or decomposition. It is soluble in cyclohexane. [Kantor et al. J Am Chem Soc 77 1685 1955, Beilstein 4 IV 4184.] |
|
Physical properties |
bp 224–224.5 °C. |
|
General Description |
In the presence of a Pd catalyst, this reagent cross-couples with aryl bromides to give styrene derivatives. |
InChI:InChI=1/C12H24O4Si4/c1-9-19(10-2)14-17(5,6)13-18(7,8)15-20(11-3,12-4)16-19/h9-12H,1-4H2,5-8H3
2554-06-5 Relevant articles
Hydrocarbyl the cyclosiloxane preparation method (by machine translation)
-
Paragraph 0019; 0033; 0040; 0041; 0045, (2017/09/20)
The invention discloses a preparation me...
Reaction of organylchlorosilanes with dimethyl sulfoxide in the presence of octamethyltrisiloxane
Basenko,Gebel',Boyarkina,Voronkov
, p. 882 - 884 (2007/10/03)
Dichloro(methyl)(vinyl)silane reacts wit...
FEATURES OF INFLUENCE OF HCl ON HYDROLYTIC COPOLYCONDENSATION OF BIFUNCTIONAL ORGANOCHLOROSILANES WITH TRIMETHYLCHLOROSILANE
Kopylov, V. M.,Agashkov, S. P.,Sunkovich, G. V.,Prikhod'ko, P. L.
, p. 1257 - 1261 (2007/10/02)
The hydrogen chloride that is formed in ...
EFFECT OF HYDROCHLORIC ACID ON RING FORMATION IN HYDROLYTIC POLYCONDENSATION OF DIFUNCTIONAL ORGANOCHLOROSILANES
Kopylov, V. M.,Agashkov, S. P.,Sunkovich, G. V.,Prikhod'ko, P. L.,Ezerets, M. A.,Glukhova, M. A.
, p. 1873 - 1878 (2007/10/02)
-
2554-06-5 Process route
-
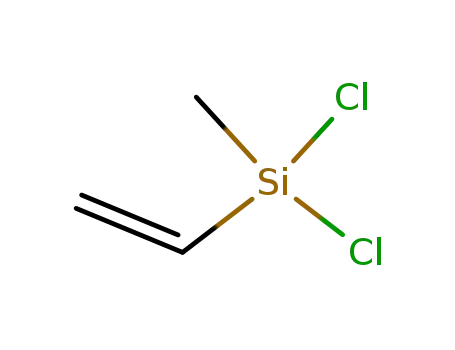
- 124-70-9
Dichloromethylvinylsilane

-
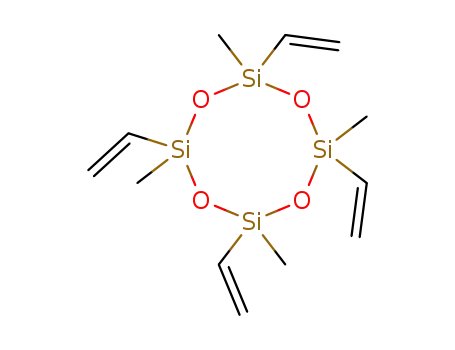
- 2554-06-5
2,4,6,8-tetramethyl-2,4,6,8-tetravinyl-cyclotetrasiloxane

-
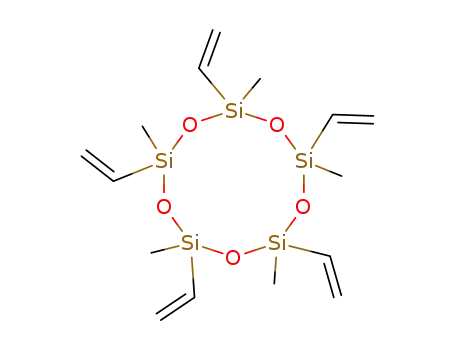
- 17704-22-2
2,4,6,8,10-pentamethyl-2,4,6,8,10-pentavinylcyclopentasiloxane
| Conditions | Yield |
|---|---|
|
With 18-crown-6 ether; water; at 20 ℃; for 0.5h; Overall yield = 90.1 %;
|
-

- 124-70-9
Dichloromethylvinylsilane

-

- 2554-06-5
2,4,6,8-tetramethyl-2,4,6,8-tetravinyl-cyclotetrasiloxane

-

- 17704-22-2
2,4,6,8,10-pentamethyl-2,4,6,8,10-pentavinylcyclopentasiloxane

-
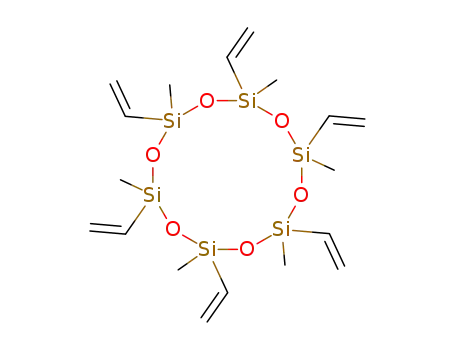
- 18304-82-0
2,4,6,8,10,12-hexamethyl-2,4,6,8,10,12-hexavinyl-cyclohexasiloxane

-
- 3901-77-7
1,3,5-trivinyl-1,3,5-trimethyl-cyclotrisiloxane
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In toluene; at 20 ℃; for 4h; Yield given. Further byproducts given. Yields of byproduct given;
|
|
|
With hydrogenchloride; In toluene; at 20 ℃; for 4h; Yield given. Yields of byproduct given;
|
2554-06-5 Upstream products
-
124-70-9

Dichloromethylvinylsilane
-
75-77-4
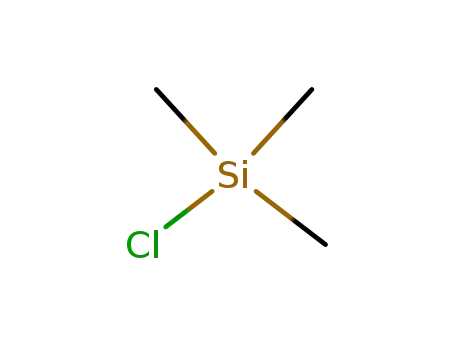
chloro-trimethyl-silane
-
5507-44-8
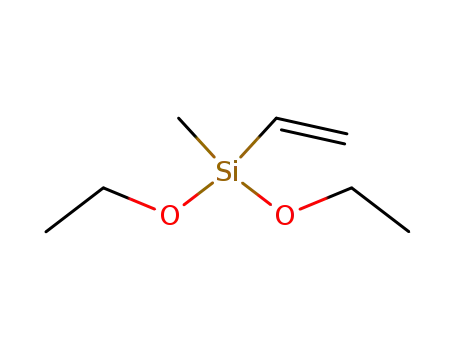
methylvinyldiethoxysilane
-
107-51-7
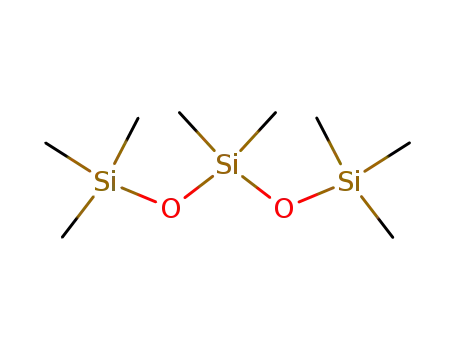
octamethyltrisiloxabe
2554-06-5 Downstream products
-
2944-70-9
vinyl methyl diacetoxysilane
-
586-39-0
1-nitro-3-vinyl-benzene
-
10537-63-0
1-(4-vinylphenyl)ethanone
-
612-15-7
o-methoxystyrene
Tetravinyl tetramethylcyclotetrasiloxane is a cyclic siloxane compound with four vinyl functional groups and four methyl functional groups. This structure makes it highly reactive in organosilicon chemistry and is commonly used in the preparation of cross-linked silicone rubber and sealants. VMC is a key raw material for high-performance silicone elastomers due to its high crosslinking density and excellent elasticity.