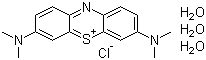
Methylene Blue trihydrate
- CAS No. :7220-79-3
- Molecular Formula:C<sub>16</sub>H<sub>24</sub>ClN<sub>3</sub>O<sub>3</sub>S
- Purity:
- Molecular Weight:373.90
Product Details
pd_meltingpoint:190 °C (dec.)(lit.)
Appearance:Dark green crystalline powder
Quality Factory Supply 99% Pure Methylene blue trihydrate 7220-79-3 with Efficient Delivery
- Molecular Formula:C16H24ClN3O3S
- Molecular Weight:373.90
- Appearance/Colour:Dark green crystalline powder
- Melting Point:190 °C (dec.)(lit.)
- PKA:2.6, 11.2(at 25℃)
- Flash Point:14°C
- PSA:75.07000
- Density:0.98 g/mL at 25 °C
- LogP:-0.69000
Methylene Blue trihydrate(Cas 7220-79-3) Usage
|
Application |
Methylene blue is not an officially restricted drug. It is mainly used to control water mildew and small melon insects. It can replace malachite green. Methylene blue dissolved in water does little harm to fish. In addition, the drug has made achievements in many fields such as industry, medicine and skin care. Even the drug will be applied to more difficult cancer diseases. Methylene blue is more effective in the treatment of non advanced fish diseases. Methylene blue is an oxidant, which can oxidize nitrite into nitrate, inhibit and kill bacteria. However, it also has a certain impact on nitrifying bacteria, so some measures should be taken after use. Methylene blue can be used in the manufacture of ink and lakes and the dyeing of biological and bacterial tissues. It can be used for dyeing cotton, hemp, silk fabrics, paper and bamboo and wood. It can also be mixed with crystal violet and yellow dextrin in the ratio of 78:13:9 to form alkaline magenta blue. |
|
General Description |
Odorless or almost odorless dark green crystals with bronze luster or dark green crystalline powder. pH (1% solution in water) 3 to 4.5 . |
|
Air & Water Reactions |
Hygroscopic. Water soluble. Gives a blue solution in water. |
|
Reactivity Profile |
Methylene Blue trihydrate is incompatible with strong oxidizing agents, caustics, alkali metal iodides and reducing agents. Also incompatible with dichromates. Methylene Blue trihydrate forms double salts with many inorganic salts. Is bleached reversibly by (zinc + hydrochloric acid) or sodium hydrosulfite. In aqueous solution, Methylene Blue trihydrate is decolorized by zinc dust and dilute sulfuric acid, but the color is restored upon exposure to air and more rapidly upon addition of ammonium hydroxide. |
|
Fire Hazard |
Flash point data for Methylene Blue trihydrate are not available; however, Methylene Blue trihydrate is probably combustible. |
InChI:InChI=1/C16H18N3S.ClH.3H2O/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13;;;;/h5-10H,1-4H3;1H;3*1H2/q+1;;;;/p-1
Trimethylene blue trihydrate, a dark blue crystalline powder. In the medical field, it is commonly used to treat diseases such as methemoglobinemia. In chemical experiments, it can be used as an oxidation-reduction indicator and a biological staining agent. For example, it is used to detect the activity of cells and the progress of certain chemical reactions.